Form
What is the Importance in Drug Substance Development of Form Screening, Polymorph Landscape, Hydrates, Amorphous State ?
What is the Importance in Drug Substance Development of Form Screening, Polymorph Landscape, Hydrates, Amorphous State ?
How is the Form Made? Crystallization can impact Form as well as material quality attributes. Milling can also impact form.
Particle size and quality influence material properties and bioavailability. Excipients and processing are factors impacting form, stability or manufacturablity.
Triform Sciences can provide technical consulting in CMC areas that are critical to integrating development of drug substance with drug product to reduce risk and accelerate clinical product manufacture.
Triform Sciences is a Pharmaceutical Sciences and Development consulting service formed by Dr. Paul Luner, a 25+ year Pharma Industry scientist with extensive experience in drug substance solid form/polymorphism, solid state and physical characterization as well as drug product development for small molecules. The company’s objective is to provide guidance and scientific direction to smaller companies seeking “Big Pharma” experience in the CMC areas where solid form/polymorphism and solid state characterization, drug substance manufacture, particle size, and drug product formulation/performance intersect.
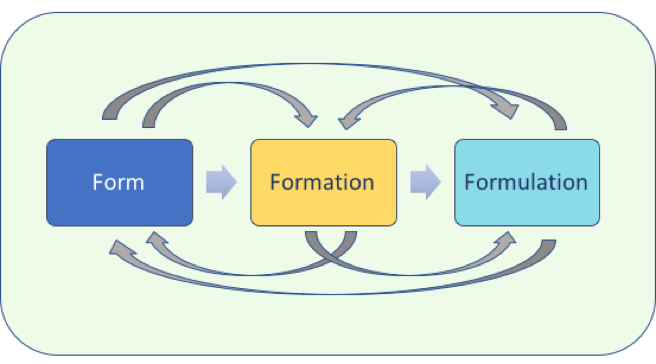
Triform Sciences was envisioned and formed based on Dr. Luner’s experience integrating the interrelationships among Form (obtaining the desired solid form/polymorph), Formation (the process to make the form and its impact on material properties and quality) and Formulation (how the form, its material properties, its potential variability impact drug product manufacturing, performance and quality) during the development cycle process to achieve QbD. Appropriate attention during the development process to the three ‘Forms’ and their interactions which impact final drug product performance can help speed development, anticipate regulatory issues, avoid significant resource requirements at later stages and achieve higher quality products.
Triform Sciences can assist with your development project by providing wholistic consulting expertise, working closely with you and your CRO(s) to find and characterize the best form for development, identify and address isolation and form scale up issues, and establish understanding and performance of the form in the formulation. We can also provide expert guidance for solving existing problems in each of these areas, design phase appropriate technical studies to address scientific or regulatory needs for efficient and cost-effective compound progression, assist in data interpretation, and support accessing technically suitable CROs.